Rocket Main Tank: Difference between revisions
page refactoring, more about insulation heat transmission and single divided tank |
→Cryogenic fuel tanks: evaporation rate |
||
| Line 19: | Line 19: | ||
Cryogenic and also low boiling temperature liquids like nitrous oxide are persistently evaporating at ambient temperature. It's like having water at 100°C and providing always more heat to it. | Cryogenic and also low boiling temperature liquids like nitrous oxide are persistently evaporating at ambient temperature. It's like having water at 100°C and providing always more heat to it. | ||
When the vapour pressure is high enough, and when tanks are solid enough too, the evaporation can reach equilibrium and the tank can contain a stable mix of liquid and gas at high pressure. This is the case for nitrous oxide at temperatures below 36.4°C, its critical temperature above which it turns all into gas, no matter what pressure is used. The issue then becomes the density of the mixture, which drops greatly. | When the vapour pressure is high enough, and when tanks are solid enough too, the evaporation can reach [https://en.wikipedia.org/wiki/Evaporation#Evaporative_equilibrium equilibrium] and the tank can contain a stable mix of liquid and gas at high pressure. This is the case for nitrous oxide at temperatures below 36.4°C, its critical temperature above which it turns all into gas, no matter what pressure is used. The issue then becomes the density of the mixture, which drops greatly. | ||
For [[LOX]], the critical temperature is -118.59°C, and the critical pressure is 50.43 bar. There's no point in keeping it so much pressurized because it could just boil off at this temperature. Since the phase change occurs at a constant temperature, we can as well choose a temperature and a pressure at which the LOX density is high enough, but that's a trade-off with the evaporation rate. Since the temperature difference between inside and outside the tank is greater, even more heat is transferred to the LOX, and more evaporation is created. Tank insulation is then required to avoid venting all the propellant before actually using it (balloon or aircraft launch can take some time to get to the launch altitude). | For [[LOX]], the critical temperature is -118.59°C, and the critical pressure is 50.43 bar. There's no point in keeping it so much pressurized because it could just boil off at this temperature. Since the phase change occurs at a constant temperature, we can as well choose a temperature and a pressure at which the LOX density is high enough, but that's a trade-off with the evaporation rate. Since the temperature difference between inside and outside the tank is greater, even more heat is transferred to the LOX, and more evaporation is created. Tank insulation is then required to avoid venting all the propellant before actually using it (balloon or aircraft launch can take some time to get to the launch altitude). | ||
A material has a [https://en.wikipedia.org/wiki/Thermal_conductivity thermal conductivity] ''k'' (unit: W/m.K), representing its ability to conduct heat. An insulation layer has a [https://en.wikipedia.org/wiki/R-value_(insulation) thermal resistance] (R-value) and its opposite, the [https://en.wikipedia.org/wiki/U-value#U-value thermal transmittance] (U-value), indicating how much resistance to heat the material provides. For an insulation layer of thickness ''L'', ''R = L/k'' and ''U = k/L''. | ===Calculating evaporation rate=== | ||
A material has a [https://en.wikipedia.org/wiki/Thermal_conductivity thermal conductivity] ''k'' (unit: W/m.K), representing its ability to conduct heat. An insulation layer has a [https://en.wikipedia.org/wiki/R-value_(insulation) thermal resistance] (R-value) and its opposite, the [https://en.wikipedia.org/wiki/U-value#U-value thermal transmittance] (U-value), indicating how much resistance to heat the material provides. For an insulation layer of thickness ''L'', ''R = L/k'' and ''U = k/L''. Unit of U is W/m^2.K. | |||
The heat transfer by convection is then given by the formula: Φ = A × U × (T1 - T2), in Watt. The thermal transmittance of the tank material can be ignored for first approximations. A is the area where the heat exchange is made, the outer surface of the insulation layer. T1 is the convecting air temperature, T2 is external tank temperature, which is close enough from the internal temperature (constant, boiling point given by pressure) to be taken for it. Examples can be found here [http://bmeweb.niu.edu.tw/pcwu/%E7%BF%92%E9%A1%8C%E8%A7%A3%E7%AD%94/Heat%20Chap01-087.doc]. | |||
Finally, the evaporation rate is the heat of vaporization ΔH<sub>vap</sub>/Φ in kg/s. That requires to know ΔH<sub>vap</sub> for the chosen storage temperature, but graphs are available for common molecules like O2. | |||
===Thermal insulation materials=== | ===Thermal insulation materials=== | ||
Revision as of 04:51, 9 November 2012
Rocket Fuel tanks
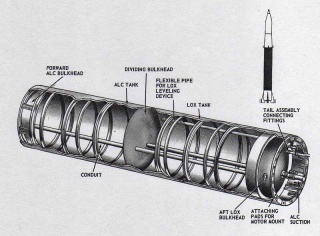
In modern launchers, two tanks are used, one for fuel and one for the oxidizer, but it has not always been the case. The Redstone rocket for example used a single tank with an internal separation, as we can see below. The sphere is the most lightweight volume (volume / area is minimized), but we can't have rockets as large as they are long, so cylinders with hemispheric caps are used. Having a single tank cut in two like for the Restone is efficient mass-wise and volume-wise but can bring new issues for insulation in case of a single cryogenic fluid (funny enough, that was the case for the Restone which used LOX and ethanol). The thicker insulation may overtake the mass benefits of a single tank.
Sloshing and other effects
Special care must be taken to avoid sloshing and vortexes in the tanks, that may lead to bubbles in propellant flow.
Wall thickness and material
Tank material first has to be stable with what's inside. Lists are available for cryogenic liquids at least. Besides this basic filter, the material choice mostly depends on money and on what's available on the market. For pressurized tanks, we will use 6061 aluminium or steel.
The thickness of the tank walls obviously depend on the internal pressure, but also on the diameter of the tank. See [1]. For example, aluminium walls can be 2mm thick and 0.4m wide for a pressure up to 13 bar with no safety factor. For a 0.2m wide tank, the thickness can be 1mm for the same pressure, or twice the pressure for the same thickness.
Cryogenic fuel tanks
Cryogenic and also low boiling temperature liquids like nitrous oxide are persistently evaporating at ambient temperature. It's like having water at 100°C and providing always more heat to it.
When the vapour pressure is high enough, and when tanks are solid enough too, the evaporation can reach equilibrium and the tank can contain a stable mix of liquid and gas at high pressure. This is the case for nitrous oxide at temperatures below 36.4°C, its critical temperature above which it turns all into gas, no matter what pressure is used. The issue then becomes the density of the mixture, which drops greatly.
For LOX, the critical temperature is -118.59°C, and the critical pressure is 50.43 bar. There's no point in keeping it so much pressurized because it could just boil off at this temperature. Since the phase change occurs at a constant temperature, we can as well choose a temperature and a pressure at which the LOX density is high enough, but that's a trade-off with the evaporation rate. Since the temperature difference between inside and outside the tank is greater, even more heat is transferred to the LOX, and more evaporation is created. Tank insulation is then required to avoid venting all the propellant before actually using it (balloon or aircraft launch can take some time to get to the launch altitude).
Calculating evaporation rate
A material has a thermal conductivity k (unit: W/m.K), representing its ability to conduct heat. An insulation layer has a thermal resistance (R-value) and its opposite, the thermal transmittance (U-value), indicating how much resistance to heat the material provides. For an insulation layer of thickness L, R = L/k and U = k/L. Unit of U is W/m^2.K.
The heat transfer by convection is then given by the formula: Φ = A × U × (T1 - T2), in Watt. The thermal transmittance of the tank material can be ignored for first approximations. A is the area where the heat exchange is made, the outer surface of the insulation layer. T1 is the convecting air temperature, T2 is external tank temperature, which is close enough from the internal temperature (constant, boiling point given by pressure) to be taken for it. Examples can be found here [2].
Finally, the evaporation rate is the heat of vaporization ΔHvap/Φ in kg/s. That requires to know ΔHvap for the chosen storage temperature, but graphs are available for common molecules like O2.
Thermal insulation materials
Cryogenic fuel tanks benefit from being insulated, which limits vaporization or even prevents boiling.
A list of thermal conductivities is available on Wikipedia.
| Material | k (mW/m.K) | density (kg/m3) | availability,comments |
|---|---|---|---|
| PU foam | 22 | a density of 24 to 32 (1.5 to 2 LB/cu.ft) should be enough | readily available, cheap, sprayed |
| Expanded polystyrene | 32 to 38 | 40 to 15 (resp.) | readily available in boards, cheap |
| cotton | around 30 | readily available, cheap | |
| mineral insulation | around 40 | readily available, cheap | |
| neoprene | 54 | 960 | readily available, cheap, heavy |
A more precise list of low conductivity materials is available here.
Propellant lines
Pumps and Engine fuel supply pipe and valve, tank pressure sensor, fill and drain pipes and valves.
For a cryogenic fuel or a high vapour pressure fuel tank: pressure relief valve, venting valve.