Aero formulas
From NPrize
The List of elementary physics formulae on wikipedia is useful.
List of variables
| Variable | Meaning | Unit (SI) |
|---|---|---|
| γ (gamma) | Surface tension | N.m-1 (Newton per meter) |
| μ (mu) or η (eta) | Viscosity | Pa·s (Pascal second) or P (Poise, 1 Poise is 0.1 Pa.s) |
| H | Enthalpy: total energy of a thermodynamic system. | J (Joule) |
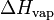 or L or L
|
Vaporization heat or Latent heat of vaporization: energy required to vaporize a mole of liquid at a given temperature. | J.mol-1 (Joule per mole) |
| Q | Amount of Heat | J (Joule) |
| T | Temperature | K (Kelvin) |
| U | Internal energy of a system (see first law of Thermodynamics below) | J (Joule) |
| V | Volume | m3 (cubic meter) |
| W | Work: mechanical constraints on the system. | J (Joule) |
| n | Quantity of matter | mol (mole) |
| p | Pressure | Pa (Pascal) |
List of constants
| Constant | Meaning | Value | Unit (SI) |
|---|---|---|---|
| NA or N | Avogadro constant, number of atoms or molecules in a mole. | 6.02214129.1023 | mol-1 |
| R | ideal gas constant | 8.3144621 | J.K−1.mol−1 |
| kB or k | Boltzmann constant, gas constant R divided by Avogadro number. | 1.3806488.10-23 | J.K-1 |
List of equations
| Equation | Name | Meaning |
|---|---|---|
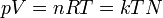
|
Ideal gas equation | Relation between properties of an ideal gas (state equation). k is kB. |
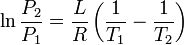
|
Clausius-Clapeyron relation | Relation between the pressure, latent heat of vaporization and temperature of a vapour at two temperatures (approximation, at low temperatures). |

|
Definition of Heat for an ideal gas. | The heat required to change the temperature of a system from an initial temperature T0, to a final temperature, Tf. |
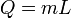
|
Heat at state change for an ideal gas. | The heat required to change the state of a some matter, L being the latent heat. Delta H equals Q only when pressure is constant (isobaric). |
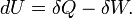
|
First law of Thermodynamics | Variations of internal energy of a system between two states is the sum of the received heat and work (minus the given work). |
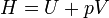
|
Enthalpy | Total amount of energy of a system, defined as the sum of the internal energy U and pressure * volume. |
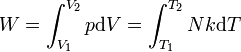
|
Work of gas expansion. | Work done by expanding an ideal gas. |