Aero formulas: Difference between revisions
From NPrize
Jump to navigationJump to search
Super scripts and fixes |
more variables and constants, images |
||
| Line 10: | Line 10: | ||
!Unit (SI) | !Unit (SI) | ||
|- | |- | ||
| | | H | ||
| | | Enthalpy | ||
| J (Joule) | |||
|- | |- | ||
| | | style="background:white; color:black"| {{SERVER}}/images/formulas_mirror/heat_vap.png or L | ||
| | | [https://en.wikipedia.org/wiki/Vaporization_heat Vaporization heat]: energy required to vaporize a mole of liquid at a given temperature. | ||
| | | J.mol<sup>-1</sup> | ||
|- | |- | ||
| T | | T | ||
| Temperature | | Temperature | ||
| K ( | | K (Kelvin) | ||
|- | |- | ||
| V | | V | ||
| Line 28: | Line 29: | ||
| Quantity of matter | | Quantity of matter | ||
| mol (mole) | | mol (mole) | ||
|- | |||
| p | |||
| Pressure | |||
| Pa (Pascal) | |||
|} | |} | ||
| Line 35: | Line 40: | ||
!Constant | !Constant | ||
!Meaning | !Meaning | ||
!Value | !Value | ||
!Unit (SI) | !Unit (SI) | ||
|- | |||
| N<sub>A</sub> or N | |||
| [https://en.wikipedia.org/wiki/Avogadro_constant Avogadro constant], number of atoms or molecules in a mole. | |||
| 6.02214129.10<sup>23</sup> | |||
| mol<sup>-1</sup> | |||
|- | |- | ||
| R | | R | ||
| [ | | [https://en.wikipedia.org/wiki/Gas_constant ideal gas constant] | ||
| 8.3144621 | | 8.3144621 | ||
| | | J.K<sup>−1</sup>.mol<sup>−1</sup> | ||
|- | |||
| k<sub>B</sub> or k | |||
| [https://en.wikipedia.org/wiki/Boltzmann_constant Boltzmann constant], gas constant R divided by Avogadro number. | |||
| 1.3806488.10<sup>-23</sup> | |||
| J.K<sup>-1</sup> | |||
|} | |||
==List of equations== | |||
{| border="1" class="wikitable" | |||
!Equation | |||
!Name | |||
!Meaning | |||
|- | |||
|style="background:white"| {{SERVER}}/images/formulas_mirror/pvnrtk.png | |||
|Ideal gas equation | |||
|Relation between properties of an ideal gas ([https://en.wikipedia.org/wiki/State_equation state equation]). | |||
|- | |||
| | |||
| | |||
| | |||
|} | |} | ||
Revision as of 02:19, 15 March 2012
The List of elementary physics formulae on wikipedia is useful.
List of variables
| Variable | Meaning | Unit (SI) |
|---|---|---|
| H | Enthalpy | J (Joule) |
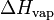 or L or L
|
Vaporization heat: energy required to vaporize a mole of liquid at a given temperature. | J.mol-1 |
| T | Temperature | K (Kelvin) |
| V | Volume | m3 (cubic meter) |
| n | Quantity of matter | mol (mole) |
| p | Pressure | Pa (Pascal) |
List of constants
| Constant | Meaning | Value | Unit (SI) |
|---|---|---|---|
| NA or N | Avogadro constant, number of atoms or molecules in a mole. | 6.02214129.1023 | mol-1 |
| R | ideal gas constant | 8.3144621 | J.K−1.mol−1 |
| kB or k | Boltzmann constant, gas constant R divided by Avogadro number. | 1.3806488.10-23 | J.K-1 |
List of equations
| Equation | Name | Meaning |
|---|---|---|
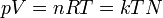
|
Ideal gas equation | Relation between properties of an ideal gas (state equation). |